Chapter 1: How To Prepare For An Organic Chemistry Experiment
Laboratory courses can be magical. They can be enlightening experiences that open your eye to a big picture. A laboratory experience should work in tandem with a lecture course, and fully realize concepts, techniques and reactions that you have heard of. Unfortunately, they can also be discouraging experiences. One of the key factors that dictates which experience you will have, is preparation.
A rewarding aspect of a well-prepared experiment is that it can firmly cement the information that you have obtained through studying in a way that is far superior to simply reading about it. Your knowledge evolves beyond routine memorizing to real understanding, because you have seen the reaction and principles with your own eyes. The synergy between a lecture course and a lab component should not be underestimated.
In this chapter I outline a systematic way of preparing for any organic chemistry experiment to ensure that you succeed in the laboratory and that you leave with an optimal experience. Seeing, after all, is believing. I discuss how to make and use the flow diagrams, how to obtain relevant safety information about the chemicals you are handling, and how to use your note-book to prepare efficiently.
Reading, Drawing And Understanding; The Power Of Visualization
There are several reasons why a systematic plan for preparation is so integral to a laboratory experience, but it boils down to the fact that an organic chemist must have information that far supersedes the information provided in a typical lab manual. When you are performing an experiment, there is little time to look up densities, perform calculations, look up safety and hazard information for chemicals and do any in-depth research on the theory behind the techniques you use. You must bring all this information with you. In many cases the lack of this information can have detrimental consequences for your experiment, your learning outcome and last but not least the safety of the laboratory.
So how can you prepare better?
Let us start by looking at the procedure. It is a recipe that describes the order of events, and the key operations that must be performed. However, just reading the procedure will never prepare you adequately.1 Why is that? The first reason, is that there is far too much material to remember, at any given time, and events that happen early in the experiment, will influence the outcome later. The second reason, is that any lab manual, even the ones designed for undergraduate use, never spells out everything that must be done. A third reason, is that any lab manual expects that the reader has some expertise and some level of experience.
This is something you most likely already know, from your everyday life.
Let’s say that you want to follow a recipe you are not familiar with to make a birthday cake. You start on step 1 and work your way through the recipe, but after a while, you run into a problem.
1 Every term we see many students that simply read the procedure (sometimes repeatedly), and end up confused and suffering through a sub-par experience.
The recipe calls for four cups of flour, but you only have two. You have a hot oven that is ready for the cake batter, a half-made frosting, and a batter that is not ready at all. You switch off the oven and rush to the store for more flour. By the time you come home, the oven is cold (and must be re-heated), and the frosting has split because you left it out in room temperature for half an hour.
This example might seem far removed from the lab, but it is perfectly transferrable. Because you had not read the recipe (or prepared adequately), you wasted time and part of your cake. Nobody wants to waste a cake.
If we take another cake-analogy and move closer to what an experiment in a lab might look like, we can say that you have a recipe for your great grandmother’s favorite chocolate cake that you wish to make. Here is the start of the recipe:
GREAT GRANDMOTHER ELSA’S FAVORITE CHOCOLATE CAKE:
- Pre-heat oven.
- Beat 1 stick of butter with 1 cup of sugar until mixture is light.
- Add 3 eggs, one at the time, to the sugar and butter mixture.
- In a bowl, add 2 cups of flour, 1 small spoon of baking powder and 1 cup of cacao-powder.
As in Elsa’s recipe and in a lab manual (even one designed for undergraduate use) we absolutely need to visualize the recipe or procedure, before we move on. You might have noted that through the act of visualizing Elsa’s recipe, I identified several issues and questions. We want to be aware of these up front, so that we have the answers and solutions before we start cooking.
Visualization is the act of seeing the procedure before your eyes, as you read it. This is a very important part of preparation, as it allows you to identify any
problems, questions or issues before you go into the lab. It also allows you time to look up valuable information. Elsa did not give us the exact temperature for baking her cake, but we could always look up what baking times for similar cakes are.
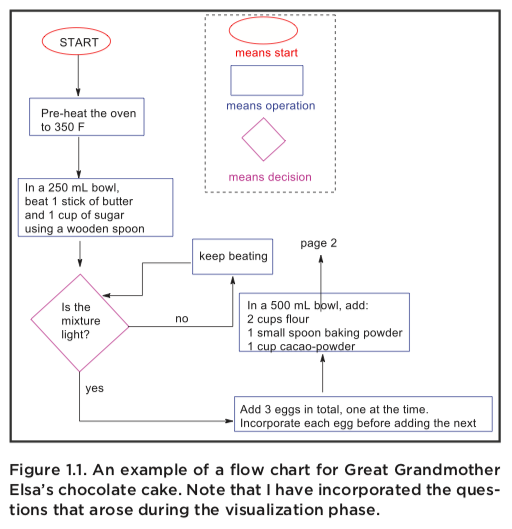
At this stage, I want to introduce another powerful tool: the flow chart. The flow chart is a graphical over- view of a procedure that should contain the essentials needed to complete the procedure. The flow chart is a product created after visualization, and it should be something you bring with you to lab. It should outline the flow of the experiment, but also provide something additional to the procedure: namely, the answers to all those questions that came up during visualization.
In the figure shown I have shown a flow chart for Great Grandmother Elsa’s recipe. Note that I have included all the information from the questions above.
I have the temperature of the oven (based on a search I did for similar cakes), I have the sizes of the bowl, and the added clarification to beat in each egg, before adding the next.
Safety Is Key- How To Find Relevant Safety Information And Read Safety Data Sheets
Safety is a number one concern for everyone working in a lab. Safety concerns have many different manifestations, and in general, we always work to make everyone as safe as possible by creating a standard procedure for whenever is in the lab. In any given situation in lab (and there are no exceptions), we use the following:
- Lab goggles. Our eyes are very delicate, and almost all chemicals are detrimental for our eye health. Goggles are worn at all times, and they are never removed in the lab. Goggles must be splash proof, which means that they protect the eyes from multiple angles, should chemicals splash.
- Lab coat. The coat is the first line of defense against chemicals that may get in contact with us, and the coat also provides a first line of defense for our clothes.
- Fume-hoods. A fume-hood is a very important part of the lab environment. It is a hood equipped with an adjustable sash, where the air flow inside the hood is very high. This removes any vapors quickly and safely. Everything that you do in an organic chemistry lab, is done in a fume-hood to minimize your exposure to fumes.
In many situations, the use of gloves is also appropriate. This is covered in detail in chapter 3.
The scary reality of working in a lab is that chemicals are dangerous. All substances have some inherent dangers associated with them, and although we spend our times exposed to many chemicals outside of the lab, the lab is a place where we can expose ourselves to a greater variety of hazardous chemicals. As a base line, we will treat any chemical as dangerous, and the utmost of care should always be taken to ensure that exposure is at a minimum. Because chemicals do vary in terms of their properties and hazards, it is also necessary to know the exact hazards of each chemical.
Where should you go to look up, identify and familiarize yourself with this information?
The answer is Safety Data Sheets (SDS).
SDS are legally required information sheets that provide an overview of the safety and hazards associated with a given chemical or mixture. There may be thousands of data sheets available for any given chemical, so before I move on, I would recommend using Sigma Aldrich as the main supplier of relevant and reliable data sheets.2
The SDS are compiled by the Globally Harmonized System of classification and labeling of chemicals (GHS), an internationally recognized system that standardizes safety and hazard information for chemicals. That means that people from all over the world should be able to access any given SDS and find the same types of information, presented in the same way.
Knowing how to work with SDS is essential for anyone working in a laboratory environment, because they contain information on the risks, hazards and safety issues associated with the chemicals in use. SDS give an informed backdrop to the experiment and helps you make safe and good choices when handling chemicals.
To contextualize this, just imagine that you were planning to handle a chemical that is explosive in contact with water. Knowing this hazard in advance is obviously key to your safety. On the other hand, what if you were planning to handle a carcinogenic substance (a substance that causes cancer)? You would obviously want to know these risks beforehand so that you could work in the safest way possible. Furthermore, if you ever plan to work in a professional laboratory, consulting SDS will be a very important part of your preparation.
2 The site http://sigmaaldrich.com/ is usually a good starting point. You can search for chemicals by name or structure.
Let us have a closer look at how a SDS is formatted.
The SDS for any given compound is typically organized into several categories, such as hazards, toxicity, composition, and first aid measures, to name a few.
The information found in a SDS can be misleading if misinterpreted. As an example, let us examine the SDS of sodium chloride (NaCl), which is table salt. Under category 4, which is first-aid information, the SDS states that if the substance is in contact with skin to “Wash off with soap and plenty of water. Consult a physician.” That seems ridiculous. I doubt that any of us call a physician if we get salt on our fingers while we are eating fries, but the restaurant cook who is dipping their fingers into a bowl of salt eight hours a day might wonder if it will cause any harmful effects. The SDS also lists information about the “acute toxicity” of sodium chloride. The data stated is LD50 Oral Rat 3,550 mg/kg. How does one interpret this?
LD50 Oral Rat refers to the amount of salt that will kill 50% of the rats who swallow it. Because rats come in all sizes, the amount is expressed as ratio between mass of compound and mass of rate. In this case, 3550 mg (or 3.55 g) of sodium chloride will kill 50% of rats who weigh 1kg.
If we assume that human bodies work like rats, we can find a lethal dose for half a population of humans. If we assume that a human weigh 100 lb (which is a convenient number) or 45.4 kg, then 45.4 x 3.55 = 161 g NaCl might be lethal to 50% of the humans who swallow it. 161 g is over 1/3 lb of salt, so the fries would probably kill you before the salt did, but there is how you interpret this figure.
As you can see from this example, the reliable information in the SDS has to be worked out ina realistic context. In this particular case, we have learned that sodium chloride can be lethal if ingested in (relatively) large amounts.
Here are some of the hazards categories that SDS frequently identify for organic chemicals, and some notes about each category to help you get started. Please note that this list is not exhaustive.
Table 1. Some common safety categories
| Category | Type | What does it mean? | Additional comments. |
| Flammable | Physical | A liquid, gas, solid or solution is flammable. Usually a spark or | Most organic liquids are flammable, so the risk of starting a fire is always present and necessary steps must always be taken to avoid such risks. |
| Oxidizing | Physical | The compound is a strong oxidant. | If you mix an oxidant with a reductant, usually a strongly exothermic reaction happens. Care should be taken to avoid this. Some oxidizers must also be disposed of in a particular way. |
| Pyrophoric | Physical | The compound ignites spontaneously in contact with air. | Pyrophoric substances are very dangerous, and usually require handling in inert gas. |
| Toxicity | Health | A substance is toxic.This category is vast, and requires specific | Most organic molecules are toxic by numerous modes. We have dedicated a separate section to toxicity below this table. |
| Skin irritation | Health | A substance irritates the skin and/ or mucus membranes | Most organic chemicals irritate the skin. If the compounds are solids, gloves often offer sufficient protection, assuming the substances do not permeate |
| Eye irritation /damage | Health | A substance irritates or permanently damages the eye | Because the eye is so delicate, most chemicals and substances damage the eye. One of the most basic safety feature of any lab, is that the people working |
| Carcinogen | Health | A substance that is suspected or known to cause cancer in mammalian cells. | This is a very important category, as substances that are either suspected or known carcinogens must be handled with extreme care. It is important to note the two subcategories of suspected and known. In practical lab work, you should deal with both these chemicals the same way: very carefully |
| Reproductive toxicity | Health | The substance damages the reproductive system | This is a very important category, as chemicals that damages the reproductive system can have very detrimental effects. |
How do you use the SDS information in your preparation phase?
- Read all SDS for all chemicals encountered in the experiment. Pay particular attention to category 2, which contains a summary of the hazard statements for the chemical.
- Analyze the information in the SDS. Think carefully about what measures can (and should!) be taken to avoid exposure, and to minimize the potential risks associated with the chemical.
- Write it down. Include the major hazard statements for each chemical in your notebook. Also, include any important analysis you have done.
A Specific SDS
Let us use all the information we have accumulated in the last sub-chapter, and use a real case example to find the relevant information we need. We will analyze the SDS of toluene, as a specific example.
We will focus on category 2 in the SDS, which is a good starting point for most SDS. This category contains hazard information. Category 2.1 contains the most important summary of the hazards and safety information associated for that chemical. For toluene, we have the following seven items:
- Flammable liquids (Category 2), H225
- Skin irritation (Category 2), H315
- Reproductive toxicity (Category 2), H361
- Specific target organ toxicity -single exposure (Category 3), Central nervous system, H336
- Specific target organ toxicity -repeated exposure (Category 2), H373
- Aspiration hazard (Category 1), H304
- Acute aquatic toxicity (Category 2), H401
Each of these items should go in the lab notebook, if toluene is used for that experiment. As a deeper analysis, we will also go through each of these seven items, and evaluate the relative hazard. As outlined in the previous sub-chapter, our aim is to analyze each item and think carefully about measures that we should take to avoid exposure and risk.
Items 1 and 2 (flammable liquid and skin irritation) are, as we have seen, typical for most organic chemicals and we follow good laboratory hygiene and work in a well ventilated fume-hood. We also wear goggles and a lab coat at all times. Item three, which deals with reproductive toxicity,3 is worth making a note of. That is an effect that we absolutely should be aware of when dealing with the chemical. Items 4 and 5 are also noteworthy. These two items say that the chemical has target toxicity both for single and repeated exposure. The first is more serious than the latter, because only one exposure of the chemical can have central nervous system toxicity. We therefore want to do everything we can to avoid contact with the chemicals. A further analysis shows that nitrile gloves offer fair protection, so we want to make sure to wear these gloves whenever handling toluene.4 Item 6 is common for most organic chemicals, as inhalation of most organic chemicals is damaging. Working in the fume-hood is one measure to limit inhalation. Item 7 is worth not- ing as well, and following proper chemical handling,5 we make sure to never pour chemicals down the drain.
The above analysis might be deeper than you are used to, but it is very important for your long-term health and well-being. One of the main goals of a chemistry lab course is to learn about safety in that environment.
3 Reproductive toxicity means a substance that in some way interfers with reproduction. It includes effects on sexual function, and fertility in both males and females, as well as developmental toxicity in the offspring.
4 Chapter 3 covers the use of gloves
5 Chapter 3 covers safe handling of chemicals
In conclusion, several safety-related issues are important for preparation.
- Find and read SDS for all chemicals used
- Find the main safety and hazard statements for each chemical used
- Include the main safety and hazard statements in your note book
The Notebook – Making Sure The Numbers Add Up
At PSU, it is required to use and keep a notebook. This notebook will be used both in the preparation of the experiment, and during the experiment itself. We will focus on how to use the notebook in the prepara- tion phase of an experiment. In chapter 4.4 we will discuss the use of the notebook during the experiment.
When stepping into the lab, your notebook should contain the following:
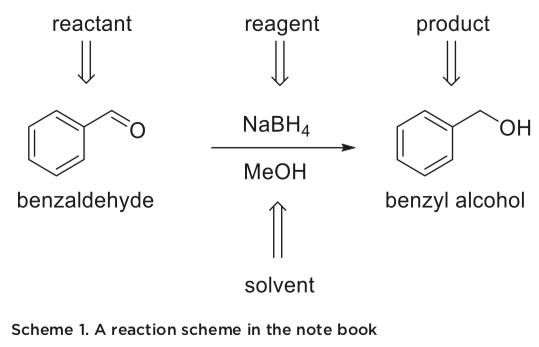
- A title and a balanced reaction scheme, if a synthesis is planned. If not, the relevant chemical structures of the compounds of interest should be shown.
- A flow-diagram that outlines the experiment, where you have included the most relevant information.
- A table or overview that shows all chemicals handled, and their risk and hazard statements.
- A synthesis table that shows the quantities of the reagents/reactants that you plan to use, with room to fill in the quantities you actually used.
- A table that shows the properties of the reagents/reactants and reaction solvents used in the procedure.
The synthesis table should contain the most important physical properties of the reactant(s), reagent(s) and product(s). This table should contain the quantities you should measure, with room to add your actual measurements. This format allows you to order all the necessary measurements in the same place. An example is shown in table 2. There are many exam- ples where this information is important in lab. If you for example measure 0.4 mL of benzaldehyde, instead of the 0.3 mL the procedure called for, you need to scale the procedure up. That means that the amount of NaBH4 also must be changed. For this process, you need the density of benzaldehyde, the molecular weight of benzaldehyde to find the moles, and the molecular weight of NaBH4.
Table 2. Example of a synthesis table for reagent(s), reactant(s) and product(s)
| Compound | Mw [g/mol] | d [g/mL] | V [mL] | m [g] | moles [mmol] | Y ield [%] |
|
Benzaldehyde NaBH4 Benzyl alcohol |
106.7 37.8 108.1 |
1.044 - 1.044 |
0.30 _____ - - |
0.31 _____ 0.15 _____ 0.21* _____ |
2.9 _____ 3.9 _____ 1.9*_____ |
- - 65.6*_____ |
* The values for benzyl alcohol is based on the procedure, that states that a yield of 65.6% is expected.
The table for physical properties is used not only for the reagent(s), reactant(s) and product(s), but also reaction solvent, work-up chemicals and others. This table is very handy in the lab, as the physical properties of the chemicals play a large part in the work-up of any reaction.
Table 3. Example of a table for physical data
| Compound | State | Boiling point [°C] | d [g/mL] | Solubility in water [g/100 mL] | Solubility in diethyl ether |
|
Benzaldehyde NaBH4 Benzyl alcohol Diethyl ether Methanol |
Liquid Solid Liquid Liquid Liquid |
178 - 205 36 65 |
1.044 Not relevant 1.044 0.71 0.79 |
0.3 Decomposes 3.50 6.05 Soluble |
Soluble Non soluble Soluble - Soluble |