Beginning Chemistry
This text introductory chemistry text is aimed for a single semester or quarter beginning experience to the field. The textmaps survey some of the basic topics of chemistry. This survey should give student enough knowledge to appreciate the impact of chemistry in everyday life and, if necessary, prepare student for additional instruction in chemistry.

Introduction
This text is disseminated via the Open Education Resource (OER) LibreTexts Project (https://LibreTexts.org) and like the hundreds of other texts available within this powerful platform, it is freely available for reading, printing and "consuming." Most, but not all, pages in the library have licenses that may allow individuals to make changes, save, and print this book. Carefully consult the applicable license(s) before pursuing such effects.
Instructors can adopt existing LibreTexts texts or Remix them to quickly build course-specific resources to meet the needs of their students. Unlike traditional textbooks, LibreTexts’ web based origins allow powerful integration of advanced features and new technologies to support learning.
The LibreTexts mission is to unite students, faculty and scholars in a cooperative effort to develop an easy-to-use online platform for the construction, customization, and dissemination of OER content to reduce the burdens of unreasonable textbook costs to our students and society. The LibreTexts project is a multi-institutional collaborative venture to develop the next generation of open-access texts to improve postsecondary education at all levels of higher learning by developing an Open Access Resource environment. The project currently consists of 14 independently operating and interconnected libraries that are constantly being optimized by students, faculty, and outside experts to supplant conventional paper-based books. These free textbook alternatives are organized within a central environment that is both vertically (from advance to basic level) and horizontally (across different fields) integrated.
The LibreTexts libraries are Powered by MindTouch® and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. This material is based upon work supported by the National Science Foundation under Grant No. 1246120, 1525057, and 1413739. Unless otherwise noted, LibreTexts content is licensed by CC BY-NC-SA 3.0.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation nor the US Department of Education.
Have questions or comments? For information about adoptions or adaptions contact [email protected]. More information on our activities can be found via Facebook (https://facebook.com/Libretexts), Twitter (https://twitter.com/libretexts), or our blog (http://Blog.Libretexts.org).
Chapter 1: What is Chemistry
What is chemistry? Simply put, chemistry is the study of the interactions of matter with other matter and with energy. This seems straightforward enough. However, the definition of chemistry includes a wide range of topics that must be understood to gain a mastery of the topic or even take additional courses in chemistry. In this book, we will lay the foundations of chemistry in a topic-by-topic fashion to provide you with the background you need to successfully understand chemistry.
Prelude to Chemistry
If you are reading these words, you are likely starting a chemistry course. Get ready for a fantastic journey through a world of wonder, delight, and knowledge. One of the themes of this book is "chemistry is everywhere," and indeed it is; you would not be alive if it were not for chemistry, because your body is a big chemical machine.

If you do not believe it, do not worry. Every chapter in this book contains examples that will show you how chemistry is, in fact, everywhere. So enjoy the ride, and enjoy chemistry.
Basic Definitions
Learning Objective
- Learn the basic terms used to describe matter
The definition of chemistry—the study of the interactions of matter with other matter and with energy—uses some terms that should also be defined. We start the study of chemistry by defining basic terms.
Matter
Matter is anything that has mass and takes up space. A book is matter, a computer is matter, food is matter, and dirt in the ground is matter. Sometimes matter may be difficult to identify. For example, air is matter, but because it is so thin compared to other matter (e.g., a book, a computer, food, and dirt), we sometimes forget that air has mass and takes up space. Things that are not matter include thoughts, ideas, emotions, and hopes.
Example 1.1:
Which of the following is matter and not matter?
- a hot dog
- love
- a tree
Solution:
- A hot dog has mass and takes up space, so it is matter.
- Love is an emotion, and emotions are not matter.
- A tree has mass and takes up space, so it is matter.
Answer: A & B.
To understand matter and how it changes, we need to be able to describe matter. There are two basic ways to describe matter: physical properties and chemical properties.
Physical Properties
Physical properties are characteristics that describe matter as it exists. Some physical characteristics of matter are shape, color, size, and temperature. An important physical property is the phase (or state) of matter. The three fundamental phases of matter are solid, liquid, and gas.

Figure 1.1 : The Phases of Matter. Chemistry recognizes three fundamental phases of matter: solid (left), liquid (middle), and gas (right). (CC BY-SA 3.0; Spirit469)
Chemical Properties
Chemical properties are characteristics of matter that describe how matter changes form in the presence of other matter. Does a sample of matter burn? Burning is a chemical property. Does it behave violently when put in water? This reaction is a chemical property as well (Figure 1.1.2). In the following chapters, we will see how descriptions of physical and chemical properties are important aspects of chemistry.
Physical Change
A physical change occurs when a sample of matter changes one or more of its physical properties. For example, a solid may melt (Figure 1.1.3), or alcohol in a thermometer may change volume as the temperature changes. A physical change does not affect the chemical composition of matter.
Chemical Change
Chemical change is the process of demonstrating a chemical property, such as the burning match in Figure 1.1.2 "Chemical Properties". As the matter in the match burns, its chemical composition changes, and new forms of matter with new physical properties are created. Note that chemical changes are frequently accompanied by physical changes, as the new matter will likely have different physical properties from the original matter.
Substance
A sample of matter that has the same physical and chemical properties throughout is called a substance. Sometimes the phrase pure substance is used, but the word pure isn't needed. The definition of the term substance is an example of how chemistry has a specific definition for a word that is used in everyday language with a different, vaguer definition. Here, we will use the term substance with its strict chemical definition.
Chemistry recognizes two different types of substances: elements and compounds.
Element
An element is the simplest type of chemical substance; it cannot be broken down into simpler chemical substances by ordinary chemical means. There are 118 elements known to science, of which 80 are stable. (The other elements are radioactive, a condition we will consider in Chapter 15.) Each element has its own unique set of physical and chemical properties. Examples of elements include iron, carbon, and gold.
Compound
A compound is a combination of more than one element. The physical and chemical properties of a compound are different from the physical and chemical properties of its constituent elements; that is, it behaves as a completely different substance. There are over 50 million compounds known, and more are being discovered daily. Examples of compounds include water, penicillin, and sodium chloride (the chemical name for common table salt).
Mixtures
Physical combinations of more than one substance are called mixtures. Elements and compounds are not the only ways in which matter can be present. We frequently encounter objects that are physical combinations of more than one element or compound—mixtures. There are two types of mixtures.
Heterogeneous Mixture
A heterogeneous mixture is a mixture composed of two or more substances. It is easy to tell, sometimes by the naked eye, that more than one substance is present.
Homogeneous Mixture/ Solution
A homogeneous mixture is a combination of two or more substances that is so intimately mixed, that the mixture behaves as a single substance. Another word for a homogeneous mixture is a solution. Thus, a combination of salt and steel wool is a heterogeneous mixture because it is easy to see which particles of the matter are salt crystals and which are steel wool. On the other hand, if you take salt crystals and dissolve them in water, it is very difficult to tell that you have more than one substance present just by looking—even if you use a powerful microscope. The salt dissolved in water is a homogeneous mixture, or a solution (Figure 1.1.3).
Chapter 2: Measurements
In 1983, an Air Canada airplane had to make an emergency landing because it unexpectedly ran out of fuel; ground personnel had filled the fuel tanks with a certain number of pounds of fuel, not kilograms of fuel. In 1999, the Mars Climate Orbiter spacecraft was lost whilst attempting to orbit Mars because the thrusters were programmed in terms of English units, even though the engineers built the spacecraft using metric units. In 1993, a nurse mistakenly administered 23 units of morphine to a patient rather than the "2–3" units prescribed (the patient ultimately survived). These incidents occurred because people were not paying attention to quantities.
Chemistry, like all sciences, is quantitative. It deals with quantities, things that have amounts and units. Dealing with quantities is very important in chemistry, as is relating quantities to each other. In this chapter, we will discuss how we deal with numbers and units, including how they are combined and manipulated.
Prelude to Measurements
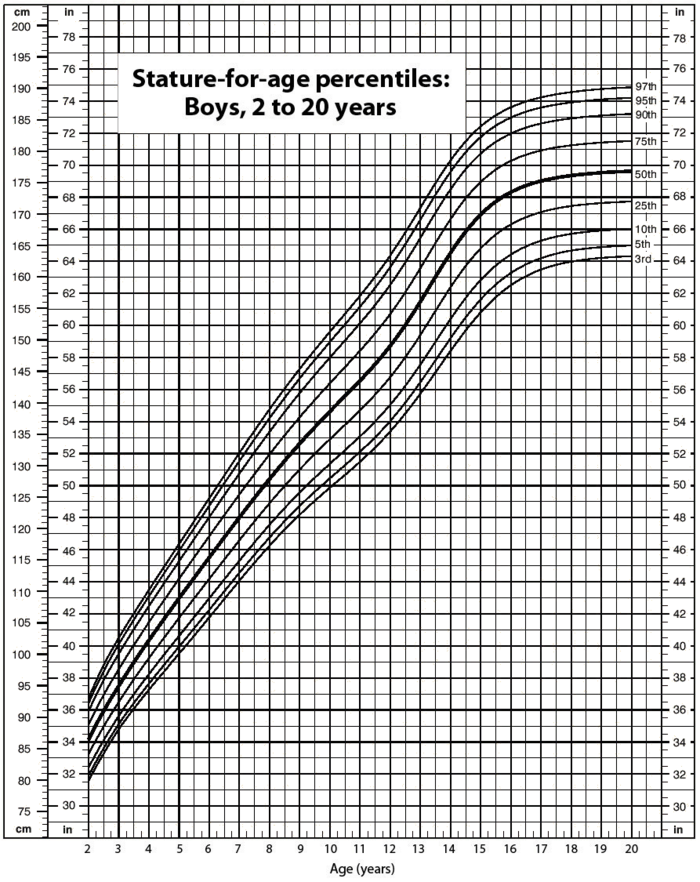
Data suggest that a male child will weigh 50% of his adult weight at about 11 years of age. However, he will reach 50% of his adult height at only 2 years of age. It is obvious, then, that people eventually stop growing up but continue to grow out. Data also suggest that the average human height has been increasing over time. In industrialized countries, the average height of people increased 5.5 inches from 1810 to 1984. Most scientists attribute this simple, basic measurement of the human body to better health and nutrition.
Expressing Numbers
Learning Objectives
- Learn to express numbers properly
Quantities have two parts: the number and the unit. The number tells "how many." It is important to be able to express numbers properly so that the quantities can be communicated properly.
Standard Notation
Standard notation is the straightforward expression of a number. Numbers such as 17, 101.5, and 0.00446 are expressed in standard notation. For relatively small numbers, standard notation is fine. However, for very large numbers, such as 306,000,000, or for very small numbers, such as 0.000000419, standard notation can be cumbersome because of the number of zeros needed to place nonzero numbers in the proper position.
Scientific Notation
An expression of a number using powers of 10.
Scientific notation is an expression of a number using powers of 10. Powers of 10 are used to express numbers that have many zeros:
| 100 | = 1 |
| 101 | = 10 |
| 102 | = 100 = 10 × 10 |
| 103 | = 1,000 = 10 × 10 × 10 |
| 104 | = 10,000 = 10 × 10 × 10 × 10 |
and so forth.
The raised number to the right of the 10 indicates the number of factors of 10 in the original number. (Scientific notation is sometimes called exponential notation.) The exponent's value is equal to the number of zeros in the number expressed in standard notation.
Small numbers can also be expressed in scientific notation but with negative exponents:
| 10−1 | = 0.1 = 1/10 |
| 10−2 | = 0.01 = 1/100 |
| 10−3 | = 0.001 = 1/1,000 |
| 10−4 | = 0.0001 = 1/10,000 |
and so forth. Again, the value of the exponent is equal to the number of zeros in the denominator of the associated fraction. A negative exponent implies a decimal number less than one.
A number is expressed in scientific notation by writing the first nonzero digit, then a decimal point, and then the rest of the digits. The part of a number in scientific notation that is multiplied by a power of 10 is called the coefficient. We determine the power of 10 needed to make that number into the original number and multiply the written number by the proper power of 10. For example, to write 79,345 in scientific notation,
dasfasdf
asdfasdf